ISO 13485 – Medical Devices Quality Management System
Ensure Safety. Enhance Quality. Achieve Compliance.
ISO 13485 is the internationally recognized standard that outlines the requirements for a Quality Management System (QMS) specific to the medical devices industry. It is designed to help organizations consistently meet regulatory requirements and customer expectations related to the design, production, installation, and servicing of medical devices and related services.
Whether you’re a manufacturer, supplier, or distributor in the medical device industry, ISO 13485 certification demonstrates your commitment to quality, safety, and compliance with global health standards.


What Is ISO 13485?
ISO 13485 is based on the structure of ISO 9001 but includes additional, sector-specific requirements for medical devices. It emphasizes risk management, product traceability, regulatory compliance, and documentation control throughout the product lifecycle—from design and development to production, distribution, and post-market activities.
This standard is often required for organizations looking to enter or expand into global medical device markets, including Europe, Canada, and other regions with strict regulatory frameworks.
WHAT ARE THE STEPS TO GET ISO 13485 CERTIFICATION ?
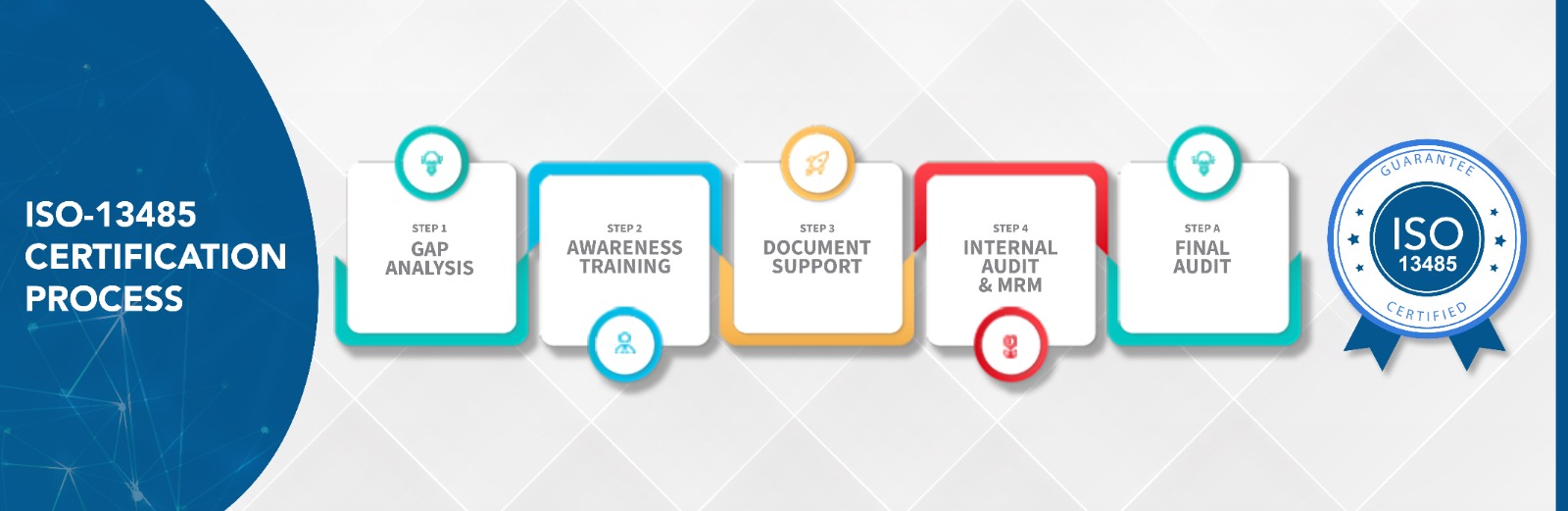
Who Needs ISO 13485 Certification?
ISO 13485 is ideal for:
Medical device manufacturers
Component and accessory suppliers
Contract manufacturers and OEMs
Medical equipment installers and servicers
Sterilization and packaging providers
Distributors and logistics partners in the healthcare sector

Who Should Be ISO 13485 Certified?
Ensure Regulatory Compliance
Align your operations with international regulatory requirements (FDA, EU MDR, Health Canada, etc.).Improve Product Safety and Effectiveness
Implement processes that reduce risk and ensure the consistent quality of medical devices.Access Global Markets
Meet the expectations of global regulators, buyers, and healthcare providers.Enhance Operational Efficiency
Streamline documentation, process control, and quality management across your organization.Boost Customer Trust
Demonstrate a strong commitment to safety, quality, and continuous improvement.Reduce Product Recalls and Non-Conformities
Apply risk-based thinking and control measures throughout your production and supply chain.
Our ISO 13485 Certification Services
We offer comprehensive support to help your organization achieve and maintain ISO 13485 certification, including:
Gap analysis and project planning
Quality manual and documentation support
Internal auditor training and awareness programs
Risk management and process validation
Mock audits and certification readiness reviews
Liaison with accredited certification bodies
Our expert consultants will work closely with your team to build a customized QMS that fits your products, processes, and regulatory needs.
Take the First Step Toward ISO 13485 Certification
With increasing scrutiny from regulators and rising expectations from healthcare providers, ISO 13485 certification is no longer optional—it’s essential.
Let us help you navigate the path to certification with ease and confidence.
Contact us today to learn more about our ISO 13485 consulting and certification support services.
